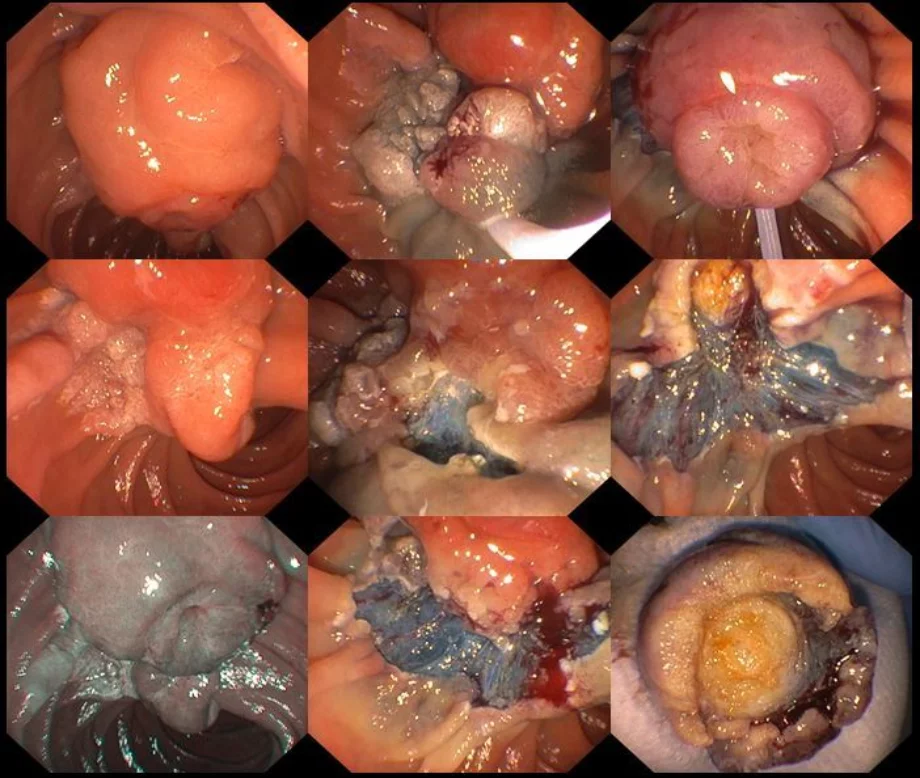
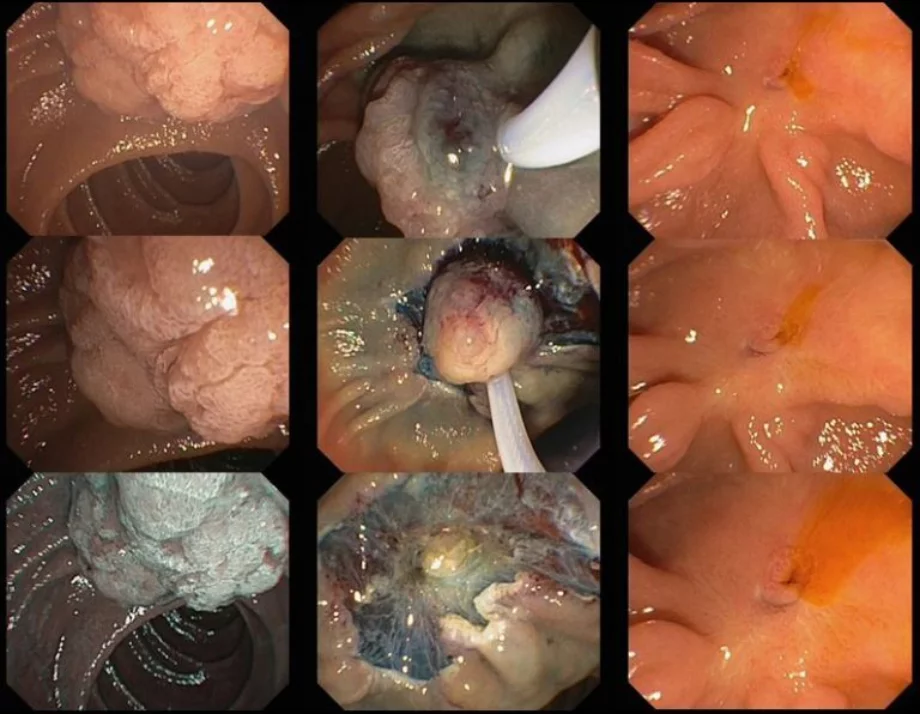
Introduction:
Sporadic and hereditary-related benign ampullary lesions can be treated endoscopically. In experienced hands, endoscopic resection is feasible and safe, resulting in long-term cure for most patients. However, adverse events such as bleeding, pancreatitis and perforation, are not uncommon, and at times may be severe. Comprehensive pre-resection evaluation, a meticulous technique with a side viewing endoscope, and experience with management of complications (mainly bleeding), are crucial to ensure a safe and adequate resection.
1. Pre-resection assessment:
Careful endoscopic assessment using high definition white light and narrow band imaging can identify lesions with advanced pathology, which may not be suitable for endoscopic resection. Biopsies may be taken from the 10-12 O’clock position of the ampulla avoiding the pancreatic orifice, which is usually at 5 o’clock. Endoscopic ultrasound, Magnetic resonance cholangiopancreatography or Computed tomography are helpful to exclude significant (>1 cm) intra-ductal extension (IDE), pan-creas divisum and other cholangiopancreatic disorders and should be performed prior to ampullectomy.
2. Scopes and Equipment:
To maximize your success, you need the appropriate equipment and an intimate knowledge of its uses. A duodenoscope is the scope of choice. Sphincterotomes, Hydrophyllic guidewires, Injection catheters, a range of stiff-thin wire snares, Coagulation forceps, Endoscopic clips, Biliary stents (plastic 10 Fr, and fully covered metal stents, 8 and 10mm diameter), Pancreatic stents (5 Fr plastic, proximal flange and single distal pig-tail), Retrieval nets.
A Microprocessor-controlled electrosurgical generator capable of delivering alternating cycles of high frequency short pulse cutting with more prolonged coagulation current is required.
3. Endoscopic technique:
Prior cholangiopancreatography is essential to evaluate for the presence of IDE, pancreas divisum and the presence of CBD dilation, before attempting resection. This is best achieved non-invasively with MRCP and can be supplemented by ERCP at the time of the procedure as necessary. Sphincterotome passage resistance or shouldered stenosis to the common bile duct (CBD) and\or pancreatic duct (PD) suggest malignant CBD and\or PD involvement; in this case, endoscopic resection should be reconsidered. Occasionally, pre-resection cannulation may be difficult. In such scenario, cannulation should be completed post resection.
Ampullectomy should only commence once complete lesion assessment has been completed. The duodenoscope should be in stable position with en face view of the ampulla. For an ampullary lesion smaller than 20mm, en bloc resection should be performed. En bloc resection may even be attempted for lesions 20mm-30mm if the adenoma does not ex-tend >1 cm beyond the papillary mound. Prior to resection you can assess the margins and feasibility for en bloc resection by moving the lesion up and down, right and left. For lesions confined to the papillary mound, no submucosal injection is needed.
When the snare is slightly open, it should be anchored at the superior margins of the ampulla and aligned to the long axis of the infundibulum with slightly to the right, for better snare control and to avoid snare dis-impaction. The snare is then slowly opened and positioned over the papilla, by gently pushing the duodenoscope distally whilst slowly opening the elevator and the snare and simultaneously with gentle force keeping the snare tip impacted in the duodenal wall above. This has been termed the fulcrum technique. Next, the snare should be closed slowly while maintaining its position parallel to the duodenal wall. When the snare is completely closed and before attempting resection, the papilla should move independently of the duodenal wall. You may check this by moving the snare back and forth with the elevator open. This maneuver is helpful to ensure suitability for resection (i.e. no invasive disease or deeper tissues captured). Once you have completed these steps, resection should be commenced. The snare should be closed completely with the elevator opened. A continuous current application of Endocut Q, effect 3 [ERBE VIO 300 (Tübingen, Germany) sometimes for 2-3 cycles, each 3-4 seconds is often required for tissue transection. Careful defect inspection should be performed immediately after the resection to ensure complete resection and exclude deep injury.
Lesions with >1 cm lateral extension onto the duodenal wall are termed lateral spreading lesions of the papilla (LSL-P) and are usually of the Paris type 0-IIa + Is. Resection of such lesions should commence with the lateral spreading component aiming to isolate the papilla for en-bloc resection. Endoscopic mucosal resection (EMR) is used to resect the lateral spreading component and previously described standard ampullectomy technique is used at the end to remove the papilla itself.
4. Pancreatic stenting
The PD should be cannulated as soon as the resection is complete. PD stenting has been reported to minimize post ampullectomy pancreatitis. We prefer using short (3-5cm) 5Fr single pigtail stent with a proximal flange. The PD orifice is identified as a slit like opening at the 5 o’clock position. Some advocate injecting a small amount of methylene blue into the PD prior to resection to facilitate identification of the orifice after resection.
5. Biliary stenting
Evidence for routine biliary stenting is lacking. Decision to place a CBD stent should be individualized for each patient. Possible advantages of biliary stenting include prevention of biliary orifice steno-sis which may predispose to cholangitis, and mitigation of bleeding and small perforations at the level of the ampullectomy. We usually prefer metallic stent 8-10mm depending on the diameter of the CBD. Biliary sphincterotomy may also be performed if there are concerns for biliary orifice stenosis.
6. Post procedure instructions
Specimens should be completely collected for pathology and ideally pinned on cork board to allow better histology assessment for lateral and deep margins. We usually keep the patients null by mouth (NBM) for 4 hours then clear fluids overnight. We give IV PPI for 24 hours followed by oral PPI twice daily for 10-12 weeks. A plain abdominal film should be obtained 7-10 days after the procedure to document PD stent migration which occurs spontaneously in 85% of cases. A CBD stent can be removed at first surveillance endoscopy 3 months after the resection.
7. Management of complications
Bleeding is the most frequent complication (10-18%) and may be severe. Intra-procedural bleeding defined as non-pulsatile continues oozing, can be treated with soft coagulation (80 Watts, Effect 4) using either the snare tip or coagulation forceps. Delayed bleeding presenting as melena in a hemodynamically stable patient can be managed with supportive treatment and observation, as usually bleeding will settle spontaneously. However, hematemesis or hematochezia in an unstable patient should prompt urgent therapeutic endoscopy utilizing standard hemostasis techniques such as injection, coagulation or clipping, all of which are significantly more difficult to perform with a duodenoscope. Angioembolization and/or surgery are rarely needed but should be considered for uncontrolled major bleeding.
8. Management of complications – Perforation:
Endoscopic diagnosis of post ampullectomy deep tissue injury is difficult. Pre-resection fluoroscopy and post procedural with the patient in the same position is very helpful for early diagnosis. When deep injury is suspected, we recommend biliary stenting with a fully covered self-expandable metallic stent. Ongoing post procedural pain should be investigated thoroughly to exclude perforation. An abdominal CT scan should be performed and a surgical consult obtained. A multidisciplinary team effort is essential to ensure the best outcome for the patient.
9. Management of complications -Pancreatitis:
We suggest aggressive pre-and intraprocedural hydration with Lactated ringer solution and administration of 100mg rectal Indomethacin to minimize risk of post-ampullectomy pancreatitis. Immediate PD cannulation and stent insertion is recommended to minimize the risk of post ampullectomy pancreatitis, except in cases of clearly documented complete pancreas divisum. Management of post ampullectomy pancreatitis follows the same principals for management of acute pancreatitis of other causes.
10. Endoscopic follow up:
Endoscopic follow up is recommended at 4-6 months and then annually for 3-5 years. Photo-documentation and biopsies should be obtained during surveillance procedures. If a recurrence is identified, it is usually diminutive and can be easily removed with snare excision or cold avulsion followed by snare tip soft coagulation.
In cases of very large, circumferential LSL-P, early endoscopic follow up at 3-4 weeks is recommended, to assess for duodenal stenosis which can be managed by balloon dilation.
Summary:
Endoscopic ampullectomy is a safe and effective therapy for papillary adenomas and LSL-P, when performed by experienced endoscopists. The optimal technique depends on lesion size and the presence and extent of extra-papillary extension along the duodenal wall. Despite its’ substantial risks and complications, these are usually mild to moderate that are conservatively managed. The endoscopist must be prepared to identify and manage these complications. It is an advanced technique that requires optimal endoscopic expertise, appropriate equipment and an experienced support team.